Abstract
Introduction: With the growing array of treatment options for multiple myeloma (MM) patients, it is important to individualize the approach at each point in the patient's treatment journey. Frailty scores such as the Revised Myeloma Comorbidity Index (R-MCI) can help match therapeutic intensity to patient fitness, particularly important in the elderly. The R-MCI has been prospectively validated in transplant patients and found to be predictive for PFS and OS outcomes. At our centre, we are enrolling patients deemed transplant-eligible by standard institutional criteria into a prospective study applying a variety of frailty tools, including objective measures of comorbidity/function and subjective measures based on patient self-assessment and physician opinion. These tools are applied at time of transplant clearance, not at diagnosis as previously studied, to take into consideration changes in frailty that occur during the initial treatment phase. The primary objective is to identify normative frailty scores at this timepoint and correlate with severity of transplant-related toxicity. In this analysis, we present data on the first 101 enrolled patients, reporting the various frailty scores and their inter-correlation.
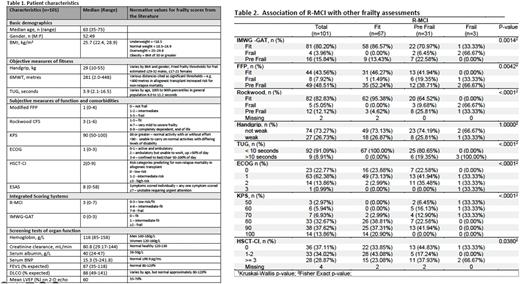
Patients and Methods: The first 101 consecutive MM patients preparing for autologous transplant (ASCT) and consented for the frailty study were included. Patients underwent comprehensive frailty assessments including: 1) objective measures of fitness [hand grip strength, 6-min walk test (6MWT), Timed Up and Go (TUG), 2) subjective measures of function (abbreviated Fried Frailty Phenotype Score (FFP), Rockwood Clinical Frailty Score, KPS, ECOG, HSCT-CI, ESAS), 3) scoring systems integrating both objective and subjective measures (R-MCI, International Myeloma Working Group geriatric assessment tool (IMWG-GAT), and 4) routine screening for organ function (creatinine clearance, serum albumin, hemoglobin, BNP, PFT, and cardiac imaging). Demographics including disease and treatment details were obtained from electronic medical records. Measures were evaluated with Chi-square/Fischer's exact test/Kruskal-Wallis test to assess the association between the various frailty scores. In addition, frailty scores were compared against R-MCI as baseline. All p values were 2-sided and p≥0.05 was considered statistically significant.
Results: From Feb 2020 to July 2022, 101 patients consented for the frailty study and were included in this analysis. Patient characteristics and frailty scores are summarised in Table 1. ASCT was part of upfront treatment in 87 patients (86%), with 14 patients (14%) receiving salvage transplant at relapse. Median age was 63 years (35-75), with 9(9%) age 70 and above. In general, our transplant-eligible population tested well on most frailty scores, mostly falling within the fit or intermediate-fit categories, as expected. Notable exceptions were significantly poorer handgrip strength and 6MWT than observed in the literature. Only 2 of 101 patients in our analysis were able to walk >400 metres, a threshold for fitness cited in prior transplant studies. Most scoring systems in our analysis correlated in designating our cohort as "fit” or "intermediate fit". When using the transplant-validated R-MCI scores as our gold standard, we observed correlations with the IMWG-GAT and Rockwood scores (p=0.0014 and <0.0001 respectively). The simple-to-perform ECOG and HCT-CI indices also correlated highly with R-MCI.(Table 2) That said, the R-MCI lacked discrimination in our patient population, with almost all patients clustered into the fit or pre-fit categories (98/101; 98%).
Conclusions: In this analysis of various frailty assessments applied at ASCT for MM, we report that most scoring systems correlated but generally lacked discrimination in this relatively fit cohort. Even the widely cited R-MCI which is validated in both myeloma and transplant, has limited utility in discriminating within this more homogeneously fit population. We will hence focus next on identifying scoring systems or individual tools that can further define the less fit or mildly frail within the "fit” category, allowing us to identify subgroups for medical optimization pre-ASCT. We also note that physical function testing such as handgrip and 6MWT may have limited utility in myeloma where disease-related disability may prevent full engagement.
Disclosures
Reece:Otsuka: Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Merck: Research Funding; BMS: Research Funding; Millenium: Research Funding; Amgen: Consultancy, Honoraria; Karyopharm: Consultancy; Sanofi: Honoraria; GSK: Honoraria. Trudel:Celgene, Amgen GSK: Consultancy; Celgene, Janssen, Amgen, GSK, Genentech: Research Funding; Celgene, Janssen, Takeda, Sanofi, Karyopharm, Amgen Canada: Honoraria. Stewart:Amgen: Consultancy; Janssen: Consultancy; Tempus Health: Consultancy, Other: Stock ownership (not including stocks owned in a managed portfolio; Sanofi: Consultancy; GlaxoSmithKline: Consultancy. Kukreti:Kwoya Kirin: Honoraria; EUSA Pharma: Honoraria. Chen:Janssen: Honoraria, Speakers Bureau; AstraZeneca: Honoraria; Gilead: Honoraria, Research Funding; BMS: Honoraria; Beigene: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal